 |
|
 |
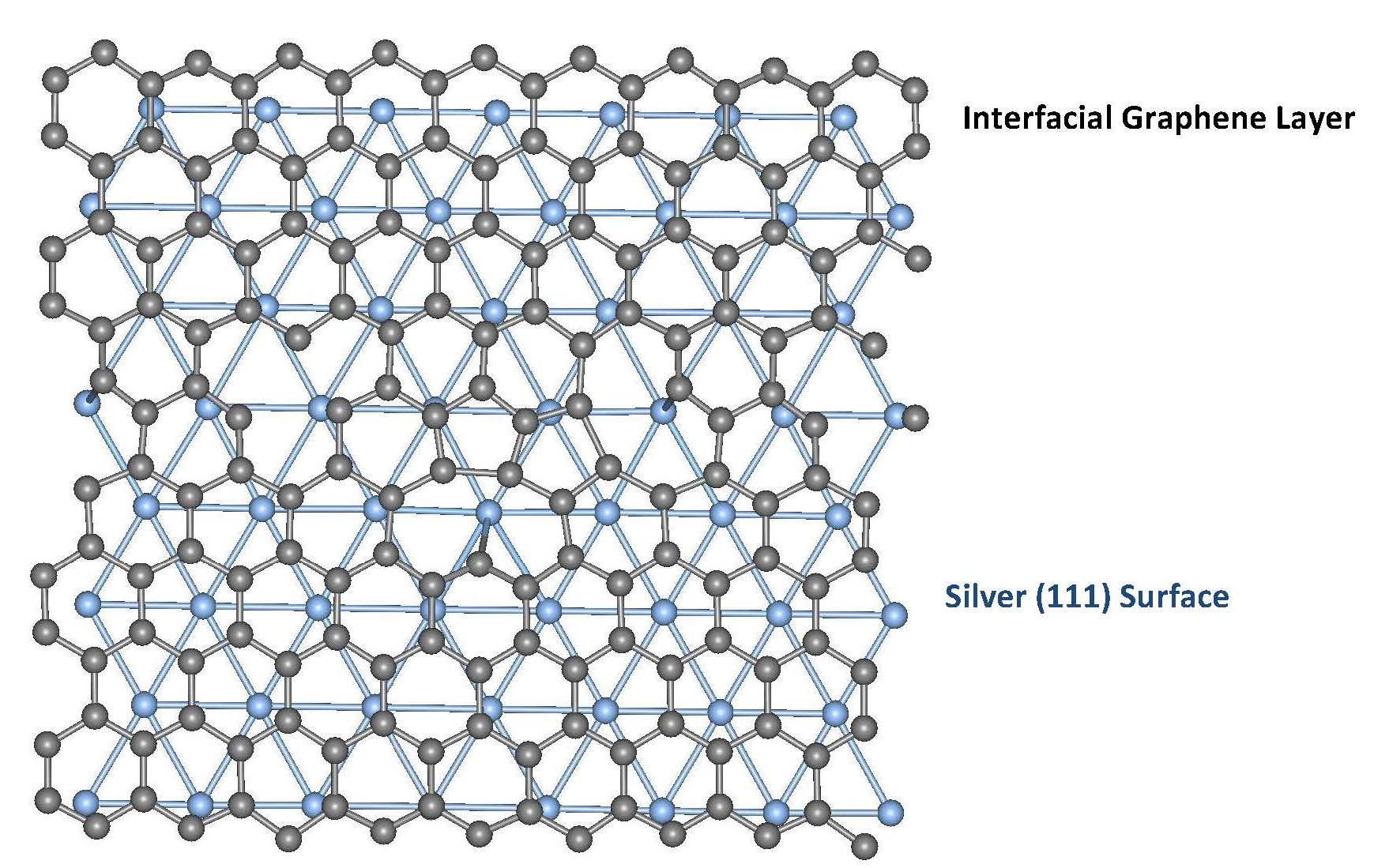
Image: Graphene layer over silver atomic planes. |
|
To meet the challenges of replacing old infrastructure and electrical grid systems, as well as upgrading aging navy vessels and aircraft, U.S. agencies such as the Department of Defense (DoD) and the Department of Energy (DOE) are currently supporting research geared towards the discovery of novel materials. The goal is find alternatives to metals that are unavailable in the U.S. thereby avoiding a dependence on materials imported from competitive, and politically unstable countries, as well as to emplace energy-efficient handling in order to meet climate change agreements.
Aluminum- and copper-based alloys are primarily used in both defense and industrial markets. Steel, a well-known carbon-based alloy, has been the key to industrial advancement in the last century – a fact that enticed scientists to explore the fusion of carbon with metals in an effort to improve durability and anti-corrosion properties. Such endeavors for mass production have been met with technical challenges. Moreover, the theoretical phase diagrams, a sort of GPS for determining the solubility of elements, suggested that for many metals (e.g. copper and silver), the solubility limit of carbon comes to parts per millions under the normal thermodynamic conditions.
Carbon was in the media spotlight when a two-dimensional form of it, known as ‘graphene,’ was discovered and became the centerpiece of research for nearly a decade due to its extraordinary mechanical and electrical properties. Indeed, Third Millennium Materials, LLC, developed a new technique to create carbon-metal bonds under non-equilibrium conditions by applying a high current to the molten metals (Al, Cu, Au, Ag, Zn, Sn, Pb and Fe) and simultaneously mixing activated carbon particles in an inert gas environment. This single-step processing of materials, called ‘covetics,’ is suitable for industrial scale production and provides increased strength, electrical and heat conductivity, as well as anti-corrosion properties in commercial aluminum-6061 and 7075 and copper.
Such significant enhancements in the properties suitable for practical applications motivated the Defense Advanced Research Projects Agency (DARPA) and the Office of Naval Research (ONR) to award contracts to UMD’s Department of Materials Science and Engineering for full scale materials characterizations by Dr. Lourdes G. Salamanca-Riba and her research group. To explore the mix of carbon and metals under the non-equilibrium conditions, advanced characterization techniques such as transmission electron microscopy (TEM), electron energy loss spectroscopy (EELS), X-ray Photoelectron Spectroscopy (XPS), Raman mapping, Atomic and Kelvin probe force microscopy (AFM and KPFM) were used in collaboration with the Army Research Lab at Aberdeen and the Naval Surface Warfare Center.
Two former graduate students, H. M. Iftekhar Jaim and Romaine A. Isaacs, discovered the existence of the epitaxial structures of carbon along different crystallographic directions of metals in nanostructure forms, like nano-ribbons and sheets. Spectrum analysis designed by machine learning (an application of artificial intelligence in signal processing) showed that the carbon-carbon bonding is sp2 bonding in aluminum, silver and copper – a highly desirable form for mimicking the properties of graphene. Unlike traditional composites where no bonding is present between the host metal and the carbon, bonding between carbon and host metals were observed in many atomic sites in covetics. Such findings are significant steps to embed 2D carbon structures in bulk metals and thin films for functionalizing the material, mechanical, and electronic properties in an energy-efficient way. The directional property of the carbon in metals is observed via the applied, high current during the production process. Moreover, non-equilibrium kinetics facilitates the carbon-metal bonding, which was mostly unknown. This process has the potential to be applied towards other elements in the periodic table for fabricating novel materials. The thin films produced by Cu-Covetic samples demonstrate the potential for better anti-corrosion properties and transparent conductors often used, for example, in solar panels and touch-screen devices.
January 30, 2017
|