 |
|
 |
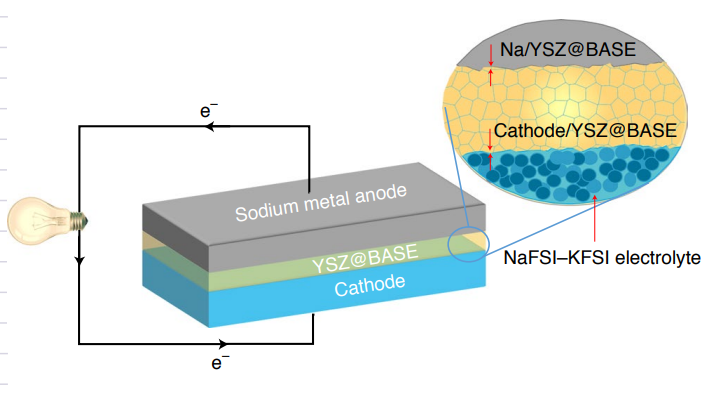
Schematic of the designed solid cell using a Na anode, YSZ@BASE electrolyte, NaFSI–KFSI electrolyte and NaNCMT/carbon-black/poly(vinyl difluoride) composite cathode. |
|
Batteries are at the heart of everything these days: communication, transportation, home goods, and so much more; thus, the need for safe, inexpensive, long-lasting batteries persists. Scientists across the globe constantly mix and match battery chemistries, looking for the perfect recipe that will fit the bill. Sodium (Na), a naturally occurring and abundant material, has been studied widely as a practical choice in next-gen energy storage. Na-batteries, having similar qualities of lithium-ion batteries, provide a safe, attractive alternative to Li-ion batteries, although Na-batteries are not without challenges. Dendrite formation, in particular, remains a hazard as does high interfacial resistance between solid electrolytes and electrodes.
To circumvent this problem, a collaborative research team at the University of Maryland (UMD) and North Carolina A&T State University, has developed an yttria-stabilized zirconia (YSZ)-enhanced beta-alumina solid electrolyte (YSZ@BASE) for low-temperature Na-batteries, which exhibit an extremely low interface impedance (3.6 Ω cm2) with the Na metal anode, while achieving an extremely high density (~7.0 mA cm–2). The study, led by Chunsheng Wang, a professor in the UMD Department of Chemical and Biomolecular Engineering (ChBE), and Xiaochuan Lu, an associate professor in the Department of Applied Engineering Technology North Carolina A&T State University, was published in Nature Nanotechnology on December 23. ChBE Ph.D. Student, Tao Deng, served as the first author.
“This battery chemistry suppresses the Na dendrite formation, while maintaining robust interfacial contacts, lower electronic conduction, and preventing the continual reduction of BASE via oxygen-ion compensation during more than 500 hours of cycling,” said Deng. “This work represents a new class of sodium batteries with a significantly lower operating temperature, which translates into a safer solid-state battery than others in the same class currently on the market.”
Keeping safety, cost and durability in mind, an operating temperature of 80 °C was selected in an effort to balance these considerations with battery performance. The team combined first-principles simulations with advanced surface characterization techniques, at which point the mechanism for enhanced performance was revealed. The YSZ phase tunes the surface chemistry and results in the formation of robust β-NaAlO2-rich solid-electrolyte interphase. At the sodiophilic phase, the YSZ also helps maintain the strong contact between electrolyte and Na anode, which is significant for the stable operation of a solid-state Na-battery.
"The aim of the research is to suppress Na dendrite growth and reduce surface resistance, both of which are critical challenges for low-temperature solid-state Li/Na-batteries,” said Wang. “We migrated these two challenges by smartly introducing the YSZ into Beta-alumina solid electrolytes. This study provides new guidance and direction for future electrolyte and interface design.”
For additional information:
Deng, T., Ji, X., Zou, L. et al (23 Dec. 2021). Interfacial-engineering-enabled practical low-temperature sodium metal battery. Nature Nanotechnology, https://doi.org/10.1038/s41565-021-01036-6
January 6, 2022
|