 |
|
 |
Self-healing chemistry based on hydrogen bonding between a hydroxyl-rich binder and an oxygen-rich active material was developed for stable Na-ion batteries. The strong chemical bonding between the binder and active material self-heals the cracks of active material upon cycling, resulting in outstanding cycling stability. |
|
Much attention has been paid to the improvement of lithium-ion batteries as of late. The cost associated with these batteries, however, will likely increase as the availability of lithium continues to decrease as manufacturing strives to keep up with demand. To mitigate this issue, researchers at the University of Maryland (UMD) Department of Chemical and Biomolecular Engineering (ChBE) – Chao Luo, Xiulin Fan, Zhaohui Ma, Tao Gao and Chunsheng Wang – are focusing on alternative energy storage systems. Sodium-ion batteries, or SIBs, are a strong candidate for an alternative power source due to the fact that sodium is so readily available, thus, cheap to manufacture.
“Extensive efforts have been devoted to developing high-capacity electrodes in SIBs such as sulfur, phosphorus, tin and organic materials,” the team reported in their research paper. “However, these high-capacity electrodes face a critical challenge because of the large volume change during sodiation and desodiation – none of electrode materials can withstand such a large volume change.” The result of this weakness is microsized particle cracking, leading to rapid battery capacity decay.
At the micro-level, ions travel back and forth between a battery’s electrodes during charge and discharge cycles. When the ions merge with the electrode material, the electrodes ‘swell’ and generate stress/strain to accommodate the ions. Once the ions leave, the electrode shrinks, but cannot return to its previous size. The accumulated stress after many charge and discharge cycles causes the electrode material to break, which means the battery fails.
But what if they could fully recover (i.e. self-heal)?
Human bone, muscle and skin are just a few biological examples of cells that can regenerate – called in-situ self-healing. Some animals, too, have amazing abilities to regenerate: lizards can regrow their tails, starfish and spiders can regrow limbs, deer can regrow antlers, and even worms and certain types of crustacean can regrow their heads if lost to a predator.
Based on these organic examples, Professor Chunsheng Wang’s research group has created a battery chemistry capable of regeneration.
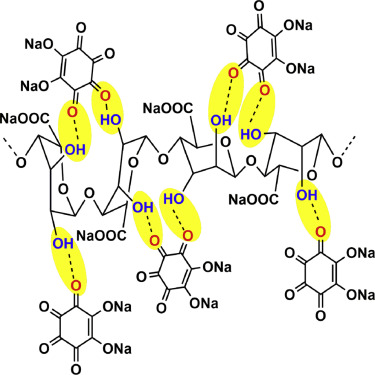
“Different from reported self-healing binder, we self-heal the active materials using binder. We have demonstrated self-healing via hydrogen bonding between hydroxyl-rich binder and microsized oxygen-rich sodium rhodizonate dibasic (SRD),” said Chao Luo, first author on the corresponding research paper.
Indeed, combining SRD with sodium-alginate (SA) created an electrode capable of regeneration, proving that the “self-healing between binder and active material paves the way for the development of high-capacity electrodes.”
“The SRD-SA electrode is capable of withstanding the microsized particle cracking during the battery cycles because the newly formed cracks in SRD particles expose oxygen from SRD molecules on the crack surface, which is able to bond with the hydroxyl-group in the SA binder,” Chao Luo added. “The large pressure generated during volume expansion of the SRD at sodiation state helps the SA binder to climb into the cracks of SRD particles, and then the hydroxyl groups in SA can chemically combine with oxygen on the crack surface through hydrogen bond to self-heal the cracks in SRD-SA electrode and maintain the electrochemical activity of cracked SRD particles. In this way, even though particle cracking occurs in SRD-SA electrode, the self-healing chemistry between binder and active material regenerates the electrode integrity and survives the high-capacity electrode.”
For additional information:
Chao Luo, Xiulin Fan, Zhaohui Ma, Tao Gao and Chunsheng Wang. “Self-healing Chemistry between Organic Material and Binder for Stable Sodium-Ion Batteries.” CHEM 3, 1-13; December 14, 2017. DOI: 10.1016/j.chempr.2017.09.004
Related Articles:
Researchers’ Battery Breakthrough Improves Performance at Lower Costs
Maryland Engineers Get Cracking on Sustainability with Crab Shell-based Battery
Building Energy Innovation in Maryland
New government partner joins UMD’s Center for Research in Extreme Batteries
ARL to Fund $30M in Equipment Innovations for Service Members
University of Maryland leads team awarded $7.2M from Army Research Lab
UMD Research Team Advances the Battery Revolution
UMD researcher receives new $1M Vehicle Technology Award
Sulfur Provides Promising 'Next-Gen' Battery Alternative
UMD Researchers Design ‘Open’ Lithium-ion Battery
December 15, 2017
|