 |
|
 |
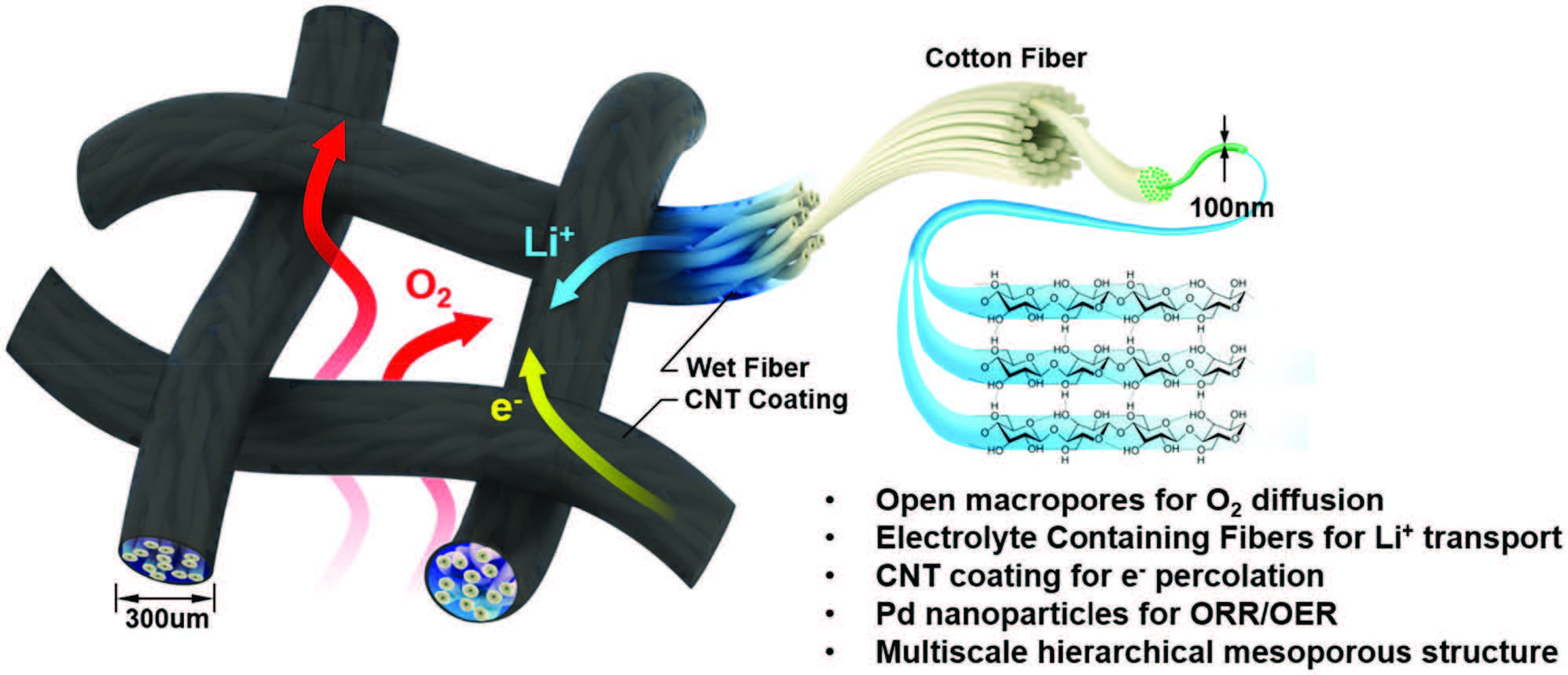
Schematic representation of the design and decoupled pathways (O2 gas, electrolyte) within the wet textile-based air cathode. The structure of the air cathode facilitates mass and ionic transport: O2 gas flows through the woven mesh, fast Li+ transport along the textile fibers, and rapid electron transfer along the CNT-coated textile surface. The coated textile, composed of intertwined nanofibers and microchannels, acts as a nanoionic device by facilitating both electrolyte and ionic (Li+) transport. |
|
Many battery researchers agree that the lithium air (Li-O2) battery is one of the most promising ‘next-gen’ energy storage devices available – more than ten times as powerful as the typical Li-ion battery due to its ultrahigh energy density. However, in conventional porous carbon air cathodes, the oxygen and electrolyte often compete for transport pathways, which limit Li-O2 battery performance.
To solve this problem, researchers in the Department of Material’s Science and Engineering (MSE) at the University of Maryland (UMD) have developed a novel textile-based air cathode, with a triple-phase structure, to improve overall battery performance. The fundamental design of fabric – layers of interwoven thread, be they natural (e.g. silk, wool or cotton) or synthetic (e.g. nylon or polyester), which form billions of tiny, square holes in a common cotton t-shirt – was the inspiration for this research.
“The woven textile-based air cathode has an inherently open structure and enables separate transport pathways for the electrolyte and oxygen gas: the electrolyte transfers along the textile fibers while the oxygen gas can diffuse through the woven mesh holes,” said MSE Postdoc, Shaomao Xu, first author on the corresponding research paper. “Due to this noncompetitive transport design, the entirety of the air cathode is accessible to both electrolyte and oxygen gas.”
What’s more, this textile-based air cathode (made of a cotton-polyester blend) is incredibly flexible – “exhibiting remarkable, mechanical strength” – which means it could easily be utilized in wearable battery design.
Many current research efforts have sought to improve lithium air battery performance via the use of solid state and hybrid electrolytes.
“More stable cathode materials have been utilized to avoid side reactions associated with electrode decomposition; active heterogeneous catalysts have been introduced to improve the kinetics of the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) during the charge/discharge processes; and redox mediators have enabled a solution-based reaction mechanism to resolve the insulating nature of the discharge products,” Dr. Xu continued. “Very few reports, however, discuss the effect of tuning cathode architecture. To our knowledge, this study is the first to use a decoupled oxygen/electrolyte pathway enabled by the unique textile structure, offering a new direction of Li-air battery research.”
For additional information:
S. Xu, Y. Yao, Y. Guo, X. Zeng, S. D. Lacey, H. Song, C. Chen, Y. Li, J. Dai, Y. Wang, Y. Chen,B. Liu, K. Fu, K. Amine, J. Lu, L. Hu. “Textile Inspired Lithium-Oxygen Battery Cathode with Decoupled Oxygen and Electrolyte Pathways.” Advanced Materials, 8Dec2017. https://doi.org/10.1002/adma.201704907
Related Articles:
Researchers’ Battery Breakthrough Improves Performance at Lower Costs
Maryland Engineers Get Cracking on Sustainability with Crab Shell-based Battery
Building Energy Innovation in Maryland
New government partner joins UMD’s Center for Research in Extreme Batteries
ARL to Fund $30M in Equipment Innovations for Service Members
University of Maryland leads team awarded $7.2M from Army Research Lab
UMD Research Team Advances the Battery Revolution
UMD researcher receives new $1M Vehicle Technology Award
Sulfur Provides Promising 'Next-Gen' Battery Alternative
UMD Researchers Design ‘Open’ Lithium-ion Battery
January 9, 2018
|